HIT Perspectives
Subscribe
HIT Perspectives – August 2025
Moving From Hybrid to Electronic Clinical Data Systems Reporting Methods: The Real-Life Payer Playbook
 By Kendra Obrist, Sr. Consultant & Payer Interoperability Expert
By Kendra Obrist, Sr. Consultant & Payer Interoperability Expert
Quick Summary
- ECDS Reporting Shift: The transition from hybrid to electronic clinical data systems (ECDS) for HEDIS® reporting is set to fully take effect by mid-year 2029.
- Key Data Sources: Payers must move from sampling member records to using validated ECDS sources like EHRs, HIEs, and clinical registries.
- Practical Playbook: This article outlines a five-phase playbook to help payers navigate the shift to ECDS, from strategy to execution.
- Long-term Success: A multiyear roadmap will guide payers to achieve full digital transformation, improving data quality, operational efficiency, and ROI.
- Partnering for Success: Health plans are encouraged to leverage trusted partners to help break down the complex transformation process into manageable steps.
Electronic clinical data system (ECDS) reporting is not a future state for Healthcare Effectiveness Data and Information Set® (HEDIS®) reporting, it is here and now is the time to get focused on it.
By mid-year 2029 (MY2029), the long-standing hybrid method of HEDIS reporting will be fully phased out. This evolution is intended to make quality measurement more accurate, timely, and cost-effective, but it comes with significant demands on health plans. Full population measurement will be required, meaning plans must use validated electronic clinical data from across the care continuum. The old playbook of sampling member records and abstracting charts is being replaced by ECDS sources defined as:
-
Electronic health records (EHRs)
-
Health information exchanges (HIEs)
-
Clinical registries
-
Case management systems
-
Administrative claims systems
As outlined in our earlier blog, The Path to Digital Quality: Why Payers Must Rethink Their Data Strategy Now, this is not just a technology challenge. Understanding “what” is required is only half the battle; figuring out the “how” is foundational to success.
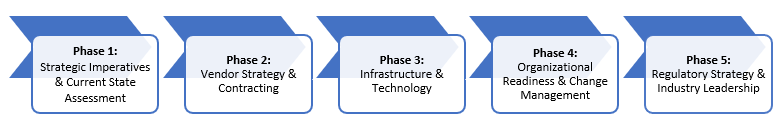
This article offers a practical, high-level playbook for payers preparing for the shift to ECDS. It covers the tactical and operational changes required to move from theory to execution in five phases:
- Strategic imperatives and assessment of current state
- Vendor strategy and contracting
- Infrastructure and technology

- Organizational readiness
- Regulatory strategy and participation in industry activity
Whether you're already headed down the ECDS path or still working through foundational questions about data sources, quality, and completeness, this playbook aims to help payer organizations turn broad digital requirements into concrete, actionable steps.
The Real-Life Playbook: From Hybrid to ECDS
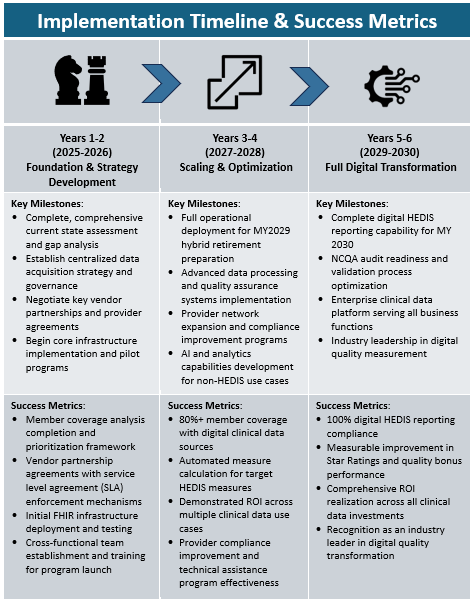
Transitioning from hybrid to electronic quality reporting methods is a significant undertaking. It’s not just about adopting new tools but also laying a data and operational foundation that enables accurate, automated, and efficient reporting. The following playbook outlines the strategic and tactical steps health plans should take to stay on track for the National Committee for Quality Assurance’s (NCQA) transition to full ECDS reporting by mid-2029, and to derive broad enterprise value from this effort.
Phase 1: Strategic Imperatives & Current State Assessment
The shift to digital quality measurement creates a compelling reason to rethink how payers acquire and manage clinical data. Instead of fragmented efforts driven by individual departments (e.g., HEDIS, risk adjustment, prior authorization), plans should adopt a centralized, enterprise-wide data strategy grounded in a "prep once, use many" philosophy. This enables better return on investment (ROI) and operational efficiency across the board.
Plans should start by defining the full scope of member health data, including claims, EHR data, labs, social determinants of health, behavioral health, and more. Then they need to evaluate their current ability to access, store, and use these data across all formats, including legacy standards and emerging Fast Healthcare Interoperability Resources® (FHIR®)-based infrastructure. A multiyear roadmap should account for both adoption of FHIR and the continued need to integrate legacy non-FHIR formats.
This phase also requires an honest assessment of current member data coverage, quality, and infrastructure readiness, as well as a financial analysis of revenue at risk (e.g., Medicare Advantage Star Ratings), current costs (manual abstraction, sampling), and the potential ROI of digital infrastructure investments. Understanding where you are—as well as where your gaps—is essential to building a pragmatic, forward-looking plan.
Phase 2: Vendor Strategy & Contracting
Payers must move from passively requesting clinical data to actively securing it through smart contracting, clear expectations, and diversified sourcing strategies. This includes revisiting provider contracts to clarify data-sharing obligations and aligning those obligations with regulatory tools like information blocking rules and Centers for Medicare & Medicaid Services (CMS) data access requirements.
Direct connections with EHR vendors may provide the fastest path to broad coverage, especially for high-value lines of business. They can be expensive and come with operational complexity, so vendor engagement must be intentional and informed by business priorities. Simultaneously, partnerships with HIEs, data aggregators, and qualified health information networks (QHINs) can help fill gaps and expand coverage cost effectively—especially as the Trusted Exchange Framework and Common Agreement (TEFCA) matures.
Equally important is provider engagement. Outreach campaigns, incentives, and technical assistance are critical for driving adoption. Providers need to understand not just the mandated changes but the value to them and their patients. Where provider participation is limited, contingency strategies such as abstraction backups or alternative data sources should be planned to protect measure completeness and mitigate risk.
Phase 3: Infrastructure & Technology
Behind every successful digital quality program is a strong technical foundation. This starts with building a scalable FHIR infrastructure and clinical data repository capable of supporting real-time ingestion, longitudinal record creation, and integration with other enterprise systems. Plans must also implement secure application programming interface gateways and ensure support for both modern and legacy formats during the transition.
Quality measurement depends on data integrity. This means investing in automated validation, normalization, and deduplication processes to clean incoming data at scale. Provenance tracking, audit trail documentation, and NCQA-aligned metadata management are essential to support both accuracy and compliance.
Though artificial intelligence (AI) can't currently be used for HEDIS measure calculation, it can be deployed to improve data quality and readiness. Natural language processing, predictive analytics, and anomaly detection can help identify missing or mismatched data, prioritize provider outreach, and prepare for future advances in quality measurement.
Phase 4: Organizational Readiness & Change Management
Technology alone won’t transform quality reporting—people and processes must evolve too. Payers need to redefine internal roles and capabilities to reflect the shift from manual, sample-based workflows to real-time, full population digital measurement.
New roles may include clinical data architects, integration specialists, and data stewards with deep knowledge of FHIR, NCQA specifications, and audit requirements. Cross-functional collaboration is also key. Information technology, quality, compliance, and analytics teams need shared fluency in standards, data strategy, and regulatory mandates.
To make the shift stick, change management must be deliberate. That includes executive alignment, metrics that demonstrate value beyond HEDIS compliance, and regular communication to sustain momentum. Ultimately, the goal is a cultural transformation—from siloed, reactive reporting to proactive, enterprise-wide data strategy.
Phase 5: Regulatory Strategy & Industry Leadership
Success in digital quality requires more than internal readiness, it also demands active participation in shaping external rules and standards. Payers should engage with NCQA, CMS, Assistant Secretary for Technology Policy (ASTP)/Office of the National Coordinator (ONC), and industry collaboratives to advocate for practical, scalable approaches to digital measurement, including improvements to programs like Data Aggregator Validation (DAV).
At the same time, payers must prepare to assert their rights under federal regulations. This means having internal processes to document and escalate information blocking concerns, enforce contractual obligations, and hold vendors accountable for unreasonable data access barriers.
Those who lead, not just comply, will be best positioned to influence evolving standards, reduce future costs, and strengthen their competitive position in an increasingly data-driven healthcare environment.
Closing Thoughts: From Overwhelm to Action
Even with a clear playbook, the reality of executing a digital quality transformation can seem overwhelming. The scope is broad. The stakes are high. Dependencies across systems, stakeholders, and data domains are anything but simple.
The good news? You don’t have to go it alone.
Health plans navigating this shift can and should lean on trusted partners to help break the work down into manageable, prioritized steps. External experts offer the objectivity needed to assess current-state capabilities, recognize overlooked opportunities, and guide decisions about where to start and where to invest next.
At Point-of-Care Partners (POCP), we’ve supported a wide range of payer clients through digital transformation efforts. Our experience includes improving data quality and governance, conducting internal skills assessments, prioritizing strategic initiatives, and leading targeted educational campaigns to support organizational change. Whether you're already deep into implementation or just starting to understand what digital quality means for your team, we can objectively help bring clarity, structure, and momentum. We often find that having an outside, independent third party helps different groups with respectively different priorities to accomplish an objective of this importance.
If you’d like to share your plans and get a fresh perspective, contact us to set up some time to chat. We would love to help.
The path forward may be complex, but you don’t have to navigate it alone. With the proper expertise and a practical plan, your organization can turn digital quality from a compliance burden into a strategic advantage.
Let’s get to work—together.




