HIT Perspectives
Subscribe
HIT Perspectives – November 2023
Navigating the Nexus: Deciphering the Interplay of Federal and State Health IT Policies
 By Kim Boyd, Regulatory Resource Center Lead
By Kim Boyd, Regulatory Resource Center Lead
Quick Summary
-
Understanding Federal vs. State Health IT Policies: Discover the nuanced influence of state-level health IT policies alongside federal regulations, illustrating their significant impact on shaping the healthcare landscape.
-
Interplay and Impact: Explore the historical relationship between state and federal policies, showcasing the ways in which states both respond to and initiate changes in health IT regulation, offering a deeper understanding of their dynamics.
-
Prior Authorization Dynamics: Delve into the intricate relationship between state and federal policies, especially in the context of prior authorization, exploring how media, consumer advocacy, and policy trends are driving notable shifts.
-
Policymaking's Effect on Stakeholders: Understand the preemptive adaptations by healthcare organizations to Notices of Proposed Rule Making (NPRMs), illustrating their proactive approach to evolving regulations.
-
Price Transparency Influence: Learn about the pivotal role price transparency plays in provider behavior at both state and federal levels, reshaping healthcare practices and fostering a more informed healthcare environment.
-
Implications of State-Level Lobbying: Explore the influence of industry organizations in shaping state-level legislation, particularly regarding prior authorization reforms, offering insights into evolving policies.
-
Navigating Complexities: Understand the challenges in harmonizing diverse state and federal health IT policies, emphasizing the necessity for stakeholders to stay informed and collaborate across state lines.
-
Call to Action: Embrace the need for stakeholders to remain proactive, informed, and engaged across federal and state policy landscapes to enhance the coherence and patient-centric nature of healthcare IT systems.
New health information technology (health IT) policies are always on the horizon and the intricate relationship between federal and state policies serves as a pivotal and often underappreciated factor. While many stakeholders tend to fixate on federal legislation and the rules set forth by entities like the Centers for Medicare & Medicaid Services (CMS) and Office of the National Coordinator for Health IT, there is a compelling argument for a shift in perspective. State-level health IT policies wield a profound influence on the health IT arena, shaping its contours and impacting the behavior of key stakeholders that include payers, providers and technology vendors.
Moreover, federal and state health IT policies go far beyond legal frameworks. They shape how stakeholders allocate their resources, posing potentially enormous ramifications for the industry's development and the quality of care delivered to patients. As current policies evolve and new ones emerge, stakeholders must reevaluate their strategies and invest in technology and infrastructure that align with the evolutions and variability present in state policies. The allocation of resources is not just a matter of compliance; it's a strategic response to the changing winds of healthcare delivery, one that has a profound impact on the efficacy of healthcare nationwide.
The Push-and-Pull Relationship Between State and Federal Policy
The interplay between state and federal health IT policy is a historical and ever-evolving dynamic. This push-and-pull relationship has existed since the nation's inception. Often, state policy activity follows the enactment of a major federal law or rule, serving to tailor, clarify or sometimes restrict how stakeholders within their respective states operate. Developments like electronic prior authorization (ePA) and prescription drug monitoring programs serve as perfect examples of how states add a layer of specificity to federal rules addressing unique state needs and nuances.
The relationship, however, isn't purely reactive. There are instances when states act before federal policy changes are implemented. This proactive approach is often driven by state-specific issues that require immediate attention. States can, therefore, act as incubators for innovative solutions and nudge federal policy in a particular direction, or they may choose to bolster enforcement where federal policy may lack the necessary teeth. The ongoing dialogue between state and federal policies is a testament to the dynamism of health IT regulation in the United States, ensuring that it remains adaptable, responsive and in tune with the diverse needs associated with the delivery of care.
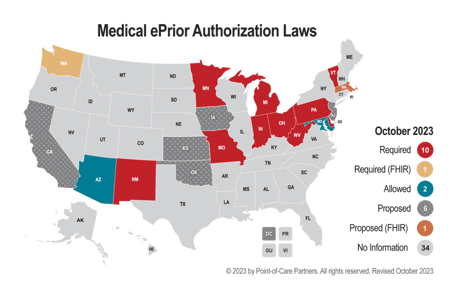
 Industry organizations like America’s Health Insurance Plans (AHIP) recognize the power of state policy, as evidenced by its response to recent proposed federal regulations with aspects of prior authorization (PA) for healthcare items and services, including providing electronic access to PA information for patients and providers, facilitating electronic exchanges of PA requests and decisions and promoting interoperability between payers. AHIP released a call to states to defer legislative or regulatory actions regarding prior authorization and align with the forthcoming federal standards to ensure consistency and uniformity, ultimately promoting more efficient and transparent healthcare processes benefiting both patients and providers.
Industry organizations like America’s Health Insurance Plans (AHIP) recognize the power of state policy, as evidenced by its response to recent proposed federal regulations with aspects of prior authorization (PA) for healthcare items and services, including providing electronic access to PA information for patients and providers, facilitating electronic exchanges of PA requests and decisions and promoting interoperability between payers. AHIP released a call to states to defer legislative or regulatory actions regarding prior authorization and align with the forthcoming federal standards to ensure consistency and uniformity, ultimately promoting more efficient and transparent healthcare processes benefiting both patients and providers.
Prior Authorization: A Case Study in Policymaking and Media Pressure
The realm of prior authorization for treatments covered by both pharmacy and medical benefits is one area in which the relationship between state and federal health IT policies has significant implications. Federal policies, often driven by agencies like CMS, lay the groundwork for how PA is handled at the national level. States, however, have their own authority in adopting policies to suit their unique healthcare environments. These state-level variations can profoundly impact healthcare stakeholders.
Consider the State of Washington's move to adopt the Fast Healthcare Interoperability Resources (FHIR) standard for automated prior authorization before the federal rule was finalized. This decision, while forward thinking, presented a challenge since it bundled both medical and prescription prior authorizations under the FHIR framework. The catch here is that ePAs for prescription medications have long been facilitated by the National Council for Prescription Drug Programs SCRIPT standard – one that's not only recognized in many states but also at the federal level. While trade associations like AHIP are certainly influential in the realm of prior authorization policy, the influence of media attention and consumer advocacy cannot be overstated. PA has long been a subject of debate, often highlighted by the media because of the administrative burdens it imposes on healthcare providers and the delays in care it causes patients. An American Medical Association (AMA) survey revealed that healthcare providers spend an average 14 hours per week navigating PA requests, with a staggering 88% categorizing the burden as high. Such revelations in the media have brought this hot-button issue to the forefront of water-cooler conversations.
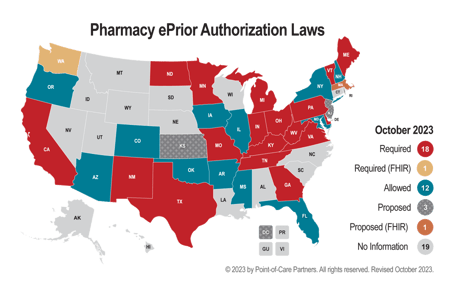 A practice called "gold carding" is also gaining traction. This entails exempting from prior authorization requirements those healthcare providers already having a track record of PA approvals, thus acknowledging their consistent adherence to established standards of care. According to a nationwide survey conducted by AHIP, the use of gold carding for medical services increased from 32% in 2019 to 58% in 2022, and from 9% to 21% for prescription medications during the same period. Some states are considering requiring gold carding. For more information, you can read our recent blog.
A practice called "gold carding" is also gaining traction. This entails exempting from prior authorization requirements those healthcare providers already having a track record of PA approvals, thus acknowledging their consistent adherence to established standards of care. According to a nationwide survey conducted by AHIP, the use of gold carding for medical services increased from 32% in 2019 to 58% in 2022, and from 9% to 21% for prescription medications during the same period. Some states are considering requiring gold carding. For more information, you can read our recent blog.
Simultaneously, consumers who frequently experience delays and barriers due to prior authorization requirements have become vocal advocates for change. Their stories and frustrations, shared through various media channels, have resonated with policymakers and the broader public, galvanizing a push for reform. This multifaceted pressure has led some healthcare payers, including UnitedHealth Group, to take proactive steps to reduce PA requirements, provoking a tangible shift in the industry's behavior.
This transformation exemplifies the power of a coordinated effort involving media scrutiny, consumer advocacy and policy trends. It highlights the capacity of these forces to push stakeholders to adapt, reform and ultimately improve the healthcare landscape, all with the goal of better serving patients and healthcare providers. The ongoing evolution of prior authorization policies underscores the potential for meaningful change within the healthcare system driven by a confluence of public, media and policy-driven pressures.
Policymaking Impacts Stakeholder Behavior from Proposed Rules to Final
The introduction of Notices of Proposed Rule Making (NPRMs) in health IT has the power to trigger a ripple effect in stakeholder behavior long before a rule is formally finalized. Healthcare organizations and technology companies often respond proactively to NPRMs, reshaping their interoperability roadmaps, resource allocation and partnership strategies. In anticipation of the impending regulations, stakeholders may initiate projects aimed at meeting the proposed requirements, ensuring they have ample time for testing and iteration to comply with the final rules once they come into effect. These early adaptations reflect the industry's recognition of the evolving regulatory landscape and the need to align their strategies, accordingly, ultimately striving to improve patient care, data sharing and the overall efficiency of healthcare systems.
It should also be noted that as of the publish date of this article, several final rules and new enforcement measures are expected to drop by the end of the year. If organizations haven’t been working against the requirements spelled out in the NRPMs that came out late last year or earlier this year, this will be a very stressful time. You can find more information on the expected rules in our recent blog.
Looking Closer at Price Transparency and Synergies Between State and Federal Policy
Price transparency plays a pivotal role in shaping the behavior of healthcare providers on both the state and federal levels. At the state level, the impact of price transparency policies on provider behavior is evident in various ways. States like Colorado, Tennessee, Maine and Texas have been at the forefront of this movement, implementing policies that demand greater cost and data transparency. Providers in these states have responded to these regulations by altering their practices to provide cost disclosure to patients. These initiatives have not only helped patients make informed decisions but also prompted providers to compete on price and quality, thereby fostering a more consumer-centric healthcare playing field.
The role of state governments in shaping price transparency legislation cannot, however, be isolated from the influence of federal guidelines. Federal agencies like CMS have taken significant steps to reduce the cost of what patients pay for prescription drugs, which in turn will have a bearing on how states formulate their price transparency policies. Federal guidelines often set the tone for state-level policy development and provide a broader framework for states to align their initiatives.
In an effort to enhance transparency and patient empowerment, state-funded websites like quality.healthfinder.fl.gov and nhhealthcost.nh.gov have been established to facilitate price comparisons for patients. These initiatives represent the synergy between state and federal endeavors to foster transparency and consumer-centric healthcare, reflecting the shared responsibility of both regulatory entities in reshaping the healthcare landscape relative to price transparency. The affiliation between state and federal actions in this domain underscores the collaborative efforts needed to create a healthcare system that is more accessible, affordable and patient focused.
Dr. Stephen Parente’s recent study on “Estimating the Impact of New Health Price Transparency Policies” highlights the significant impact policy can have on the healthcare market. In it, Parente purports that the implementation of payer price transparency rules will potentially result in substantial annual savings for consumers, employers and payers by 2025. These savings are estimated based on a comprehensive set of data sources focusing on 70 shoppable services as defined by the Department of Health and Human Services. The analysis suggests potential savings ranging from $17.6 billion to $80.7 billion for the privately insured population in the United States. The impact of price transparency varies by region and income level, with lower income individuals experiencing the most significant impact.
While these findings emphasize the potential of price transparency policies to significantly reduce healthcare costs, the distribution of these savings among consumers, employers and health plans remains to be determined. This research provides the first national estimate of the impact of price transparency and associated policies and highlights the potential to mitigate rising healthcare costs.
Industry Organization Lobbying Efforts at the State Level Should Not Be Ignored
Industry organizations like the AMA have dedicated substantial lobbying efforts to shape and promote model state-level legislation addressing the issues surrounding prior authorization. An increasing number of states are now considering legislation to address that time-consuming and care-disrupting practice. In the current legislative session, nearly 90 PA reform bills have been deliberated in 30 states, with over a dozen still pending potential approval. These bills, such as those in Arkansas, California, New Jersey, North Carolina, Washington, DC and Washington state, often draw from the AMA's model legislation. They encompass a range of PA reforms, including establishing swift response times, requiring adverse determinations to be made by state-licensed physicians of the same specialty, prohibiting retroactive denials for preauthorized care, and ensuring authorizations remain valid at least one year. Additionally, the legislation may demand transparency through the public release of payers’ PA data and mandate that new policies honor a patient's PA from a previous policy for at least 90 days. This momentum toward reform is a powerful development, with state- and federal-level initiatives gaining traction as policymakers and stakeholders evaluate the impact of prior authorization on patient care.
Balancing Federal and State Interests: A Call to Stakeholders
It’s imperative that payers and health IT vendors closely monitor and understand federal and state health IT policies. These policies have a direct bearing on how you operate and design software that's utilized in specific states. As we navigate the intricate nexus between federal and state health IT policies, it becomes evident that harmonizing these often-divergent regulations is a significant challenge.
States possess distinct healthcare landscapes and unique needs, justifying the necessity of state-led initiatives. These initiatives offer the advantage of tailoring policies to specific state requirements and effectively addressing local healthcare issues. However, the resulting patchwork of state-led policies creates complexities for stakeholders operating across state lines, be it payers, providers or health IT vendors.
Navigating the Future of Health IT
Health IT policy is in a state of perpetual evolution, shaped by technological advances, changing healthcare needs and shifting policy priorities. It's crucial to understand the dynamic impact of state actions on federal health IT priorities and vice versa. States, with their autonomy, can tailor policies to their unique contexts, and these actions can ripple through the entire healthcare industry, significantly influencing federal decision making, standards and regulations.
In this uncertain terrain, stakeholders, especially payers and health IT vendors, must remain vigilant and engaged not only at the federal level but also in proactively monitoring state-level policy proposed, passed and failed. Recognizing the interconnection of these two spheres is imperative in shaping the future of health IT. Looking ahead, the call to action is clear: stay informed, be proactive, and collaborate across state lines to create a more coherent, efficient and patient-centric healthcare IT system. Organizations can rely on resources like Point-of-Care Partners’ Regulatory Resource Center to help navigate the intricate web of state and federal health IT policy. In addition to our subscription services, our knowledgeable team can help your organization understand how policy may impact the nuances of your strategic and interoperability roadmaps. Reach out to me at kim.boyd@pocp.com to set up a time to chat.




